2019
11. Tf2O-Mediated Intermolecular Coupling of Secondary Amides with Enamines/ Ketones: A Versatile, Direct Access to b-Enaminones Yong-Peng Liu, Cheng-Jie Zhu, Cun-Cun Yu, Ai-E Wang,* and Pei-Qiang Huang,* Eur. J. Org. Chem. 2019, 7169-7174.

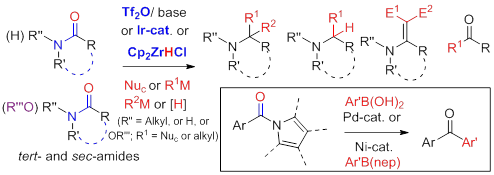
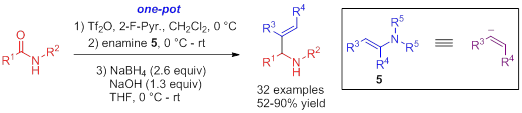
A novel approach to β‐enaminones has been developed, based on a Tf2O‐mediated reaction of secondary amides with ketones enamines. The method can be extended to the one‐pot condensation of secondary amides with ketones for β‐enaminones synthesis.
12. 光催化氧化还原体系中硝酮与芳香叔胺的自由基偶联反应 刘玉成,郑啸,* 黄培强*化学学报 2019, 77, 850-855.
Photoredox Catalysis for the Coupling Reaction of Nitrones with Aromatic Tertiary Amines Liu Yu-Cheng; Zheng Xiao; Huang Pei-Qiang Acta Chim. Sinica 2019, 77, 850-855.

13. Organocatalytic, Enantioselective Reductive Bis-Functionalization of Secondary Amides: One-pot Construction of Chiral 2,2-Disubstituted 3-Iminoindoline, Zhen Xu, Xiao-Gang Wang, Yong-Hua Wei, Kan-Lei Ji, Jian-Feng Zheng*, Jian-Liang Ye* and Pei-Qiang Huang* Org. Lett. 2019, 21, 7587-7591.

14. Cross-coupling of Secondary Amides with Tertiary Amides: The Use of Tertiary Amides as Surrogates of Alkyl Carbanions for Ketone Synthesis, Shu-Ren Wang and Pei-Qiang Huang * Chin. J. Chem. 2019, 37, 887—891. DOI: 10.1002/cjoc.201900215

15. Double Addition of Alkynyllithium Reagents to Amides/ Lactams: A Direct and Flexible Synthesis of 3-Amino-1,4-diynes Bearing an Aza-Quaternary Carbon Center, Hang Chen, Ying-Hong Huang, Jian-Liang Ye, and Pei-Qiang Huang* J. Org. Chem. 2019, 84, 9270-9281.

16. Ketone Synthesis by Direct, Orthogonal Chemoselective Hydroacylation of Alkenes with Amides: Use of Alkenes as Surrogates of Alkyl Carbanions, Hui Geng and Pei-Qiang Huang,* Chin. J. Chem. 2019, 37, 811-816. DOI: 10.1002/cjoc.201900252

17. Chemoselective Synthesis of a-Amino-a-cyanophosphonates by Reductive Gem-Cyanation-Phosphonylation of Secondary Amides, Ting-Ting Chen, Ai-E Wang,* and Pei-Qiang Huang*, Org. Lett. 2019, 21, 3808-3812. DOI: 10.1021/acs.orglett.9b01257 Publication Date (Web): May 6, 2019 (Letter) In honor of the 90th birthday of Professor Qing-Yun Chen.陈庆云

18. Biomimetic Enantioselective Total Synthesis of (–)-Robustanoids A, B and Analogs, Zhan-Jiang Liu and Pei-Qiang Huang,* J. Org. Chem. 2019, 84, 5627-5634. DOI: 10.1021/acs.joc.9b00573 Publication Date (Web): April 8, 2019 (Article) In honor of the 90th birthday of Professor Qian-Er Zhang 张乾二

19. Intermolecular Dehydrative [4 + 2] Aza-Annulation of N-Arylamides with Alkenes: A Direct and Divergent Entrance to Aza-Heterocycles, Ying-Hong Huang, Shu-Ren Wang, Dong-Ping Wu, and Pei-Qiang Huang,* Org. Lett. 2019, 21, 1681-1685. DOI: 10.1021/acs.orglett.9b00233 Publication Date (Web): February 26, 2019 In honour of the 120th birthday of the late Professor Dr. Ming-Long Huang.黄鸣龙

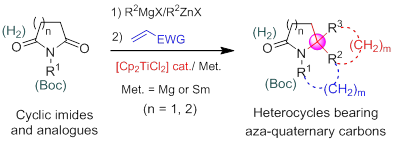
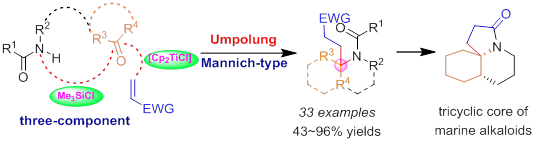
20. Construction of Multifunctional Heterocycles Bearing Aza-Quaternary Carbons by Titanocene-Catalyzed Umpolung Reactions, Jiang-Tao Cheng, Xiao Zheng,* Pei-Qiang Huang,* Tetrahedron 2019, 75, 1612-1623. Special issue In honor of the 95th birthday of the late Professor We-Shan Zhou.
21. Ir-Catalyzed Chemoselective Reduction of β-Amido Esters: A Versatile Approach to β-Enamino Esters, Zhi-Ping Yang,† Guang-Sheng Lu,† Jian-Liang Ye,* Pei-Qiang Huang*, Tetrahedron 2019, 75, 1624-1631. Special issue In honor of the 95th birthday of the late Professor We-Shan Zhou.

22. A stepwise annulation for the transformation of cyclic ketones to fused 6 and 7-membered cyclic enimines and enones, Dong-Ping Wu, Qian He, Dong-Huang Chen, Jian-Liang Ye, and Pei-Qiang Huang,* Chin. J. Chem. 2019, 37, 315-322. DOI: 10.1002/cjoc.201900035 (Breaking report) In honour of the 90th birthday of Professor Xi-Yuan Lu.

Polycyclic Structure Synthesis via a Three‐step “[2+n]” Annulation
Dong‐Ping Wu; Qian He Chinese J. Chem. 2019, 37, 529-530

23. Enamines as Surrogates of Alkyl Carbanions for the Direct Conversion of Secondary Amides to a-Branching Ketones, Yong-Peng Liu, Shu-Ren Wang, Ting-Ting Chen, Cun-Cun Yu, Ai-E Wang* and Pei-Qiang Huang,* Adv. Synth. Catal. 2019, 361, 971-975. DOI: 10.1002/adsc.201801443兰州Dedicated to Professor Hui-Lin Wan on occasion of his 80th birthday

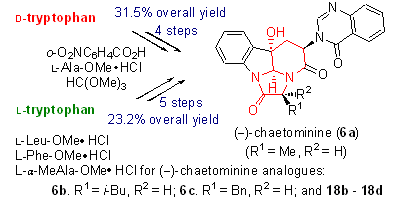
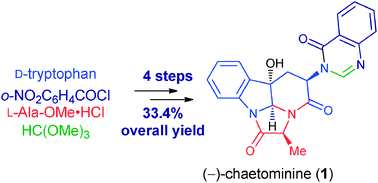
24. Rapid Generation of Molecular Complexity by Chemical Synthesis: Highly Efficient Total Synthesis of Hexacyclic Alkaloid (-)-Chaetominine and Its Biosynthetic Implications, Hui Geng, Pei-Qiang Huang,* Chem. Rec. 2019, 19, 523-533. DOI: 10.1002/tcr.201800079 In memory of Wei-Shan Zhou on the occasion of his 95th birthday
With biogenetic thinking, the hexacyclic alkaloid (−)-chaetominine has been synthesized in only four steps from d-tryptophan and in just five steps from L-tryptophan. This short-step generation of molecular complexity by chemical synthesis established several records in terms of steps, overall yields, and starting point.

2018
25. 书评:《现代天然产物化学》(王锋鹏主编,科学出版社,北京: 2009), 黄培强, 有机化学, Huang, Pei-Qiang*, Chin. J. Org. Chem. 2018, 38, 2461-2463.
26. 基于杂环的不对称插烯Mannich反应及其在生物碱合成中的应用进展, 叶剑良, 黄培强, 有机化学, Progress in Heterocycle-based Asymmetric Vinylogous Mannich Reactions and Applications to the Synthesis of Alkaloids, Huang, Pei-Qiang*, Chin. J. Org. Chem. 2018, 38, 2215-2230. (Invited to the special issue on chemical synthesis and synthetic biology)

27. Iridium-Catalyzed Reductive Alkylations of Secondary Amides, Wei Ou, Feng Han, Xiu-Ning Hu,† Hang Chen,† and Pei-Qiang Huang,* Angew. Chem. Int. Ed. 2018, 57, 11354-11358; Angew. Chem. 2018, 130, 11524-11528. DOI: 10.1002/anie.201806747. Dedicated to Professor Guo-Qiang Lin林国强 on occasion of his 75th birthday

28. A Versatile One-Pot Synthesis of Polysubstituted Cyclopent-2-enimines from α,β-Unsaturated Amides via Imino-Nazarov Reaction, Ting Fan, Ao Wang, Jia-Qi Li, Jian-Liang Ye,* Xiao Zheng,* and Pei-Qiang Huang,* Angew. Chem. Int. Ed. 2018, 57, 10352-10356. Angew. Chem. 2018, 130, 10509–10513. DOI: 10.1002/anie.201805641 and 10.1002/ange.201805641 南开

29. Asymmetric Total Synthesis and Absolute Configuration Determination of (−)-Verrupyrroloindoline, Zhi-Ping Yang, Qian He, Jian-Liang Ye,* and Pei-Qiang Huang,* Org. Lett. 2018, 20, 4200–4203. DOI: 10.1021/acs.orglett.8b01579

30. Palladium-Catalyzed [3+2]-C-C/N-C Bond Forming Annulation, Yang Liu, Zhongyi Mao, Alexandre Pradal, Pei-Qiang Huang, Julie Oble,* and Giovanni Poli,* Org. Lett. 2018, 20, 4057-4061. DOI: 10.1021/acs.orglett.8b01616
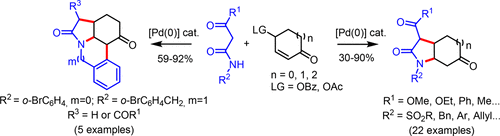
31. Iridium-Catalysed Reductive Coupling Reaction of Tertiary Lactams/Amides with Isocyanoacetates, Xiu-Ning Hu, Tai-Long Shen, Dong-Cheng Cai, Jian-Feng Zheng,* Pei-Qiang Huang,* Org. Chem. Front. 2018, 5, 2051 – 2056.
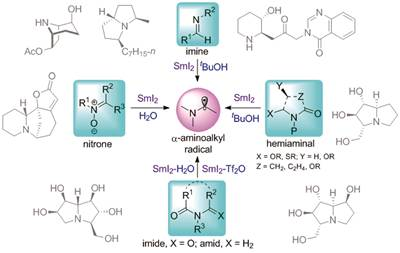
32. 二碘化钐参与及二茂钛催化的氮a-位碳自由基偶联反应及其在含氮杂环合成中的应用, 郑啸, 黄培强*, 化学进展, 2018, 30, 528-546. SmI2 and Titanocene-Mediated Coupling Reactions of a-Aminoalkyl Radicals and Applications to the Synthesis of aza-Heterocycles, Progress in Chem. 2018, 30, 528-546. Special issue In memory of Zhi-Tang Huang on the occasion of his 90th birthday
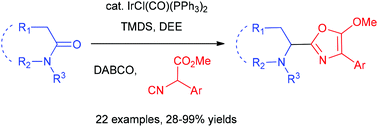
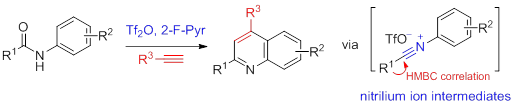
33. Metal-Free Synthesis of Quinolines by Direct Condensation of Amides with Alkynes: Revelation of N-Aryl nitrilium Intermediates by 2D NMR Techniques, Ye Jian-Liang, Zhu Ya-Nan, Geng Hui, Huang Pei-Qiang,* Sci. China-Chem. 2018, 61, 687-694. DOI: 10.1007/s11426-017-9160-1
34. 酰胺直接转化: 策略与近期进展, 黄培强, 化学学报, Direct Transformations of Amides: Tactics and Recent Progress, Huang, Pei-Qiang, Acta Chim. Sinica 2018, 76, 357-365.
35. Chemoselective Direct Reductive Trifluoromethylation of Amides: A Flexible Access to Functionalized a-Trifluoromethylamines, Hang Chen, Jian-Liang Ye,* and Pei-Qiang Huang,* Org. Chem. Front. 2018, 5, 943 – 947. DOI: 10.1039/c7qo01031a
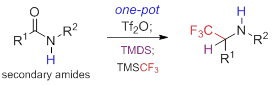
36. Enamines as Surrogates of Alkene Carbanions for the Reductive Alkenylation of Secondary Amides: An Approach to Allylamines, Ai-E Wang,* Cun-Cun Yu, Yong-Peng Liu, Pei-Qiang Huang,* Org. Lett. 2018, 20, 999-1002. DOI: 10.1021/acs.orglett.7b03943
37. Ni-Catalyzed Chemoselective Alcoholysis of N-Acyloxazolidinones, Pei-Qiang Huang,* and Hui Geng, Green Chem. 2018, 20, 593-599. DOI: 10.1039/C7GC03534A南开 祝贺田先生九十大寿
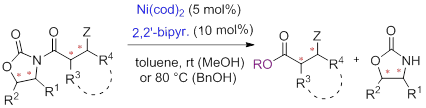
38. Dual Catalysis for Enantioselective Convergent Synthesis of Enantiopure Vicinal Amino Alcohols, Chen-Xi Ye, Yared Yohannes Melcamu, Heng-Hui Li, Jiang-Tao Cheng, Tian-Tian Zhang, Yuan-Ping Ruan, Xiao Zheng,* Xin Lu,* and Pei-Qiang Huang,* Nature Commun. 2018, 9:410. doi:10.1038/s41467-017-02698-4
Highlighted by: (1) Synform 2018/06, A96–A100; (2) Huang, Sha-Hua; Hong, Ran, Sci. China-Chem. 2018, 61, 509-510; (3) 《科学通报》(2018, 63, 1755-1757)
https://www.nature.com/articles/s41467-017-02698-4

2017
39. Ni-Catalyzed Cross-Coupling Reactions of N-Acylpyrrole-Type Amides with Organoboron Reagents, Pei-Qiang Huang* and Hang Chen, Chem. Commun. 2017, 53, 12584 – 12587. DOI: 10.1039/C7CC07457C

40. Intramolecular Keto-lactam Condensation: A Convenient and Straightforward Approach to Bicyclic Vinylogous Lactams, Pei-Qiang Huang,* and Ting Fan, Eur. J. Org. Chem. 2017, 6369–6374. DOI: 10.1002/ejoc.201701060

41. Analogues of the 2-Carboxyl-6-Hydroxyoctahydroindole (CHOI) Unit from Diverging Pd-Catalyzed Allylations: Selectivity as a Function of the Double Bond Position, Zhongyi Mao, Elisabetta Martini, Guillaume Prestat, Julie Oble,* Pei-Qiang Huang,* and Giovanni Poli,* Tetrahedron Lett. 2017, 58, 4174-4178.
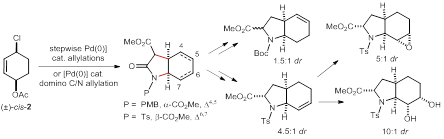
42. Substrate-Controlled Chemoselective Reactions of Isocyanoacetates with Amides and Lactams, Jian-Feng Zheng, Xiu-Ning Hu, Zhen Xu, Dong-Cheng Cai, Tai-Long Shen, and Pei-Qiang Huang,* J. Org. Chem.2017, 82, 9693–9703. DOI: 10.1021/acs.joc.7b01768
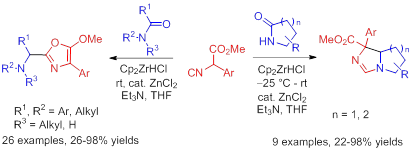
43. An Attempted Approach to the Tricyclic Core of Haliclonin A: Structural Elucidation of the Final Product by 2D NMR, Yan-Jiao Gao, Shi-Peng Luo, Jian-Liang Ye, and Pei-Qiang Huang,* Chin. Chem. Lett. 2017, 26, 1176-1181.
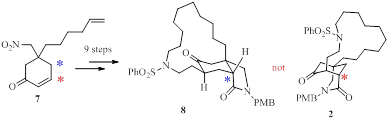
44. 含未保护羟基2-吡咯烷酮衍生物的直接还原氰基化:N-甲基-2-别-Bulgecinine的立体选择性合成, 高燕娇†,肖振华†,刘良先*,‡,黄培强*, 有机化学,Direct Reductive Cyanation of A 2-Pyrrolidinone Chiral Building Block Bearing An Unprotected Hydroxyl Group: A Stereoselective Synthesis of N-Methyl-2-epi-bulgecinine, Gao, Yan-Jiao,† Xiao, Zhen-Hua,† Liu, Liang-Xian,*,‡ Huang, Pei-Qiang*, Chin. J. Org. Chem. 2017, 37, 1189-1197. DOI: 10.6023/cjoc201703024.
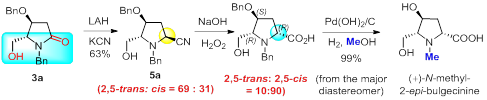
45. Further Studies on the Direct Synthesis of a,b-Unsaturated Ketimines and a,b-Enones by Chemoselective Dehydrative Addition of Functionalized Alkenes to Secondary Amides, Pei-Qiang Huang (黄培强)*, and Ying-Hong Huang (黄应红), Chin. J. Chem. 2017, 35, 613-620. DOI: 10.1002/cjoc.201600700
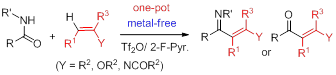
46. One-pot synthesis of N-heterocycles and enimino carbocycles by tandem dehydrative coupling–reductive cyclization of halo-sec-amides and dehydrative cyclization of olefinic sec-amides,Pei-Qiang Huang,* Ying-Hong Huang, and Shu-Ren Wang, Org. Chem. Front. 2017, 4, 431-444. DOI: 10.1039/c6qo00720a
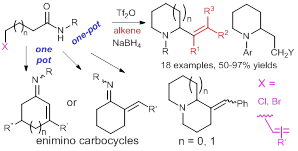
47. A Direct, Versatile, and Chemoselective Synthesis of Vinylogous Bis- and mono-urethanes/ Amides, and β-Keto Esters by Aza-Knoevenagel-type Reactions of Teriary Amides with Enolates, Pei-Qiang Huang,* and Wei Ou, Eur. J. Org. Chem. 2017, 582-592. DOI: 10.1002/ejoc.201601326 南开 In memory of the late Professor Dr. Qi-Rui Cai
2016
48. Qi-Wei Lang,and Xiu-Ning Hu,Pei-Qiang Huang,* Tf2O - TMDS Combination for the Direct Reductive Transformation of Secondary Amides to Aldimines, Aldehydes, and/ or Amines,Sci. China-Chem. 2016, 59, 1638-1644. 四区
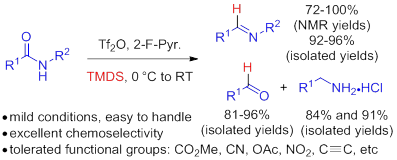
49. Pei-Qiang Huang,* Qi-Wei Lang, and Xiu-Ning Hu, One-pot Reductive 1,3-Dipolar Cycloaddition of Secondary Amides: A Two-Step Transformation of Primary Amides,J. Org. Chem.2016, 81, 10227–10235. DOI: 10.1021/acs.joc.6b01080 (Invited by C. Dale Poulter, Editor-in-Chief, and associated editors: Daniel L. Comins and Dawei Ma for special issue on Heterocycles)
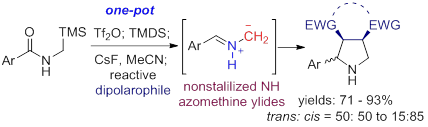
50. Pei-Qiang Huang,* Ying-Hong Huang, and Kai-Jiong Xiao, Metal-free Intermolecular Coupling of Arenes with Secondary Amides: Chemoselective Synthesis of Aromatic Ketimines and Ketones, and N-Deacylation of Secondary Amides,J. Org. Chem.2016, 81, 9020-9027. DOI: 10.1021/acs.joc.6b01647
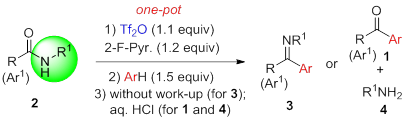
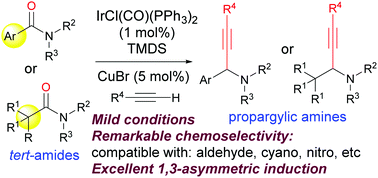
51. Pei-Qiang Huang,* Wei Ou, and Feng Han, Chemoselective Reductive Alkynylation of Tertiary Amides by Ir and Cu(I) Bis-metal Sequential Catalysis, Chem. Commun. 2016, 52, 11967—11970.
52. Ying-Hong Huang, Hui Geng, and Jian-Liang Ye, Metal-Free C–H Alkyliminylation and Acylation of Alkenes with Secondary Amides, Pei-Qiang Huang,* Sci. Rep. 2016, 6: 28801. doi: 10.1038/srep28801 (A journal published by the Nature Publishing Group). http://www.nature.com/articles/srep28801 In memory of the late Professor Wei-Yuan Huang.
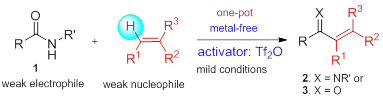
53. Pei-Qiang Huang,* Qi-Wei Lang, and Yan-Rong Wang, Mild Metal-free Hydrosilylation of Secondary Amides to Amines, J. Org. Chem.2016, 81, 4235–4243. DOI: 10.1021/acs.joc.6b0057
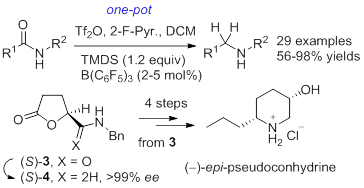
54. Jian-Liang Ye,* Yang Liu, Yu-Feng Zhang, Zhi-Ping Yang, Pei-Qiang Huang,* Studies on the Second-Generation Approach to Loline Alkaloids: Synthesis of N-Bus-norloline via N-tert-Butanesulfinyl Imine-based Asymmetric Vinylogous Mannich Reaction, Synthesis 2016, 48, 1684-1692. DOI: 10.1055/s-0035-1561432. (Invited for Special Topics Issue focused on Target Oriented Synthesis of Complex Molecules, Guest editor: Prof. Dr. Erick M. Carreira)
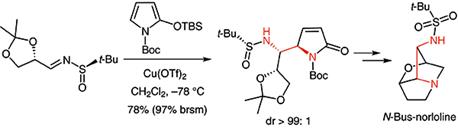
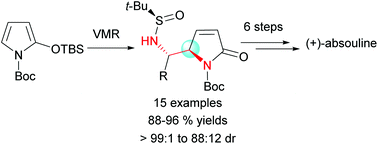
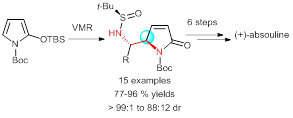
55. Jian-Liang Ye,* Hang Chen, Yu-Feng Zhang, and Pei-Qiang Huang* A Versatile Access to Vicinal Diamine Motifs by Highly anti-Selective Asymmetric Vinylogous Mannich Reactions: An Efficient Total Synthesis of (+)-Absouline, Org. Chem. Front. 2016, 3, 683-692.
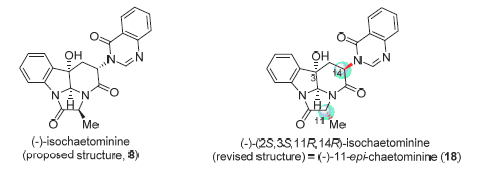
56. 黄培强,* 茅中一, 耿辉, (-)-Isochaetominine推测结构的对映选择性全合成与结构修正, 有机化学,2016, 36 (2), 315-324. Huang, Pei-Qiang,* Mao, Zhong-Yi, Geng, Hui, Enantioselective Total Synthesis and Structural Revision of (-)-Isochaetominine, Chin. J. Org. Chem. 2016, 36, 315-324. DOI:10.6023/cjoc201512015. (Invited, Cover article)
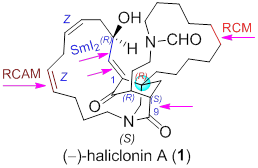
57. Lian-Dong Guo, Xiong-Zhi Huang,† Shi-Peng Luo,† Wen-Sen Cao, Yuan-Ping Ruan, Jian-Liang Ye, Pei-Qiang Huang,* Organocatalytic, Asymmetric Total Synthesis of (-)-Haliclonin A, Angew. Chem. Int. Ed. 2016, 55, 4064-4068. Angew. Chem. 2016, 128, 4132-4136. DOI: 10.1002/anie.201512005. (most accessed paper published in Angew. Chem. Int. Ed. in Feb., 2016; highlighted by Synfacts).
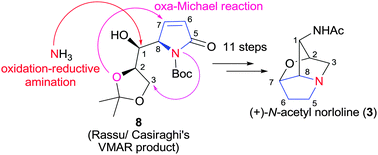
58. Jian-Liang Ye,* Yang Liu, Zhi-Ping Yang and Pei-Qiang Huang,* Asymmetric Total Synthesis of (+)-N-Acetyl Norloline, Chem. Commun. 2016, 52, 561-563. DOI: 10.1039/C5CC07480K.
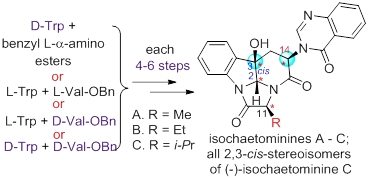
59. Zhong-Yi Mao, Hui Geng, Tian-Tian Zhang, Yuan-Ping Ruan, Jian-Liang Ye, and Pei-Qiang Huang,* Stereodivergent and Enantioselective Total Syntheses of Isochaetominines A–C and Four Pairs of Isochaetominine C Enantiomers: A Six-Step Approach,Org. Chem. Front. 2016, 3, 24-37. DOI: 10.1039/C5QO00298B.
2015
60. Xiao Zheng,* Jiang He, Heng-Hui Li, Ao Wang, Xi-Jie Dai, Ai-E Wang, Bao-Ding Zhang, Chen-Guang Qiu, and Pei-Qiang Huang, Titanocene(III)-Catalyzed Three-Component Reaction of Secondary Amides, Aldehydes, and Electrophilic Alkenes, Angew. Chem. Int. Ed. 2015, 54, 13739-13742. Angew. Chem. 2015, 127, 13943-13946. DOI: 10.1002/anie.201506907.
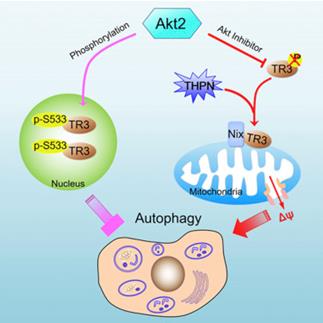
61. Wei-jia Wang, Yuan Wang, Pei-pei Hou, Feng-wei Li, Bo Zhou, Hang-zi Chen, Xue-li Bian, Qi-xu Cai, Yong-zhen Xing, Jian-ping He, Hongkui Zhang, Pei-qiang Huang, Tianwei Lin,* Qiao Wu,* Induction of Autophagic Death in Cancer Cells by Agonizing TR3 and Attenuating Akt2 Activity, Chem. & Biol. 2015, 22, 1040-1051.
62. Ai-E Wang,* Zong Chang, Yong-Peng Liu, Pei-Qiang Huang,* N-Deacylation of secondary amides by alkylation with organocerium reagents, Chin. Chem. Lett. 2015, 24, 1055-1058. DOI: 10.1016/j.cclet.2015.05.033
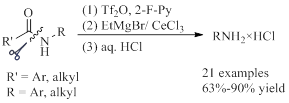
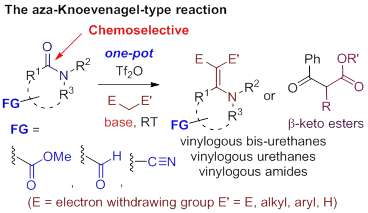
63. Pei-Qiang Huang,* Wei Ou, and Jian-Liang Ye, Aza-Knoevenagel-type Condensation of Secondary Amides: A Direct Access to N-Mono-substituted b,b-difunctionalized Enamines,Org. Chem. Front. 2015, 2, 1094 – 1106. DOI: 10.1039/C5QO00191A

64. 郑剑峰*,谢志强,陈欣健,黄培强*, 酰胺的直接转化: 仲酰胺和丹尼谢夫斯基双烯的还原环加成反应,化学学报 2015, 73, Jian-Feng Zheng,* Zhi-Qiang Xie, Xin-Jian Chen, and Pei-Qiang Huang* Direct Transformation of Amides: Reductive Cycloaddition of Secondary Amides with Danishefsky Diene, Acta Chim. Sinica 2015, 73, 705-715.
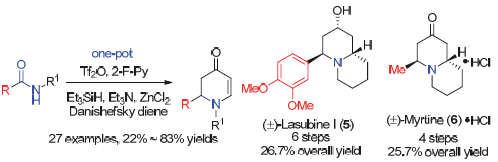
65. Jian-Feng Zheng,a* Xiang-Yang Qiana and Pei-Qiang Huanga* Direct Transformation of Amides: One-pot Reductive Ugi-Type Three-component Reaction of Secondary Amides,Org. Chem. Front. 2015, 2, 927-935. DOI: 10.1039/C5QO00146C
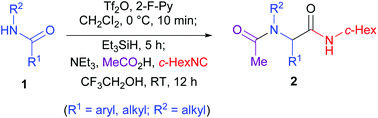
66. Shi-Peng Luo,‡ Hui Geng,‡ Yu Wang, and Pei-Qiang Huang,* Low-valent Titanium-Mediated Eantioselective Synthesis of Quinazolinone Alkaloids Circumdatins F, H, and Analogs, Chin. J. Chem. 2015, 33, 646-654. DOI: 10.1002/cjoc.201400849
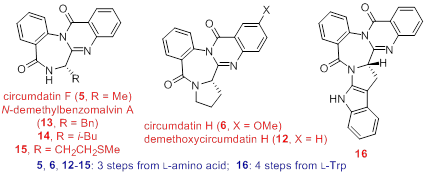
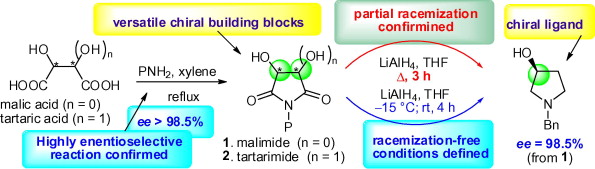
67. Pei-Qiang Huang,* Wei Ou, and Jian-Liang Ye, Towards Reaction Control: An Expeditious Access to Racemic 5-Substituted Tetramates and 5-Substituted Tetramic Acids from Malimides, Chin. J. Chem. 2015, 33, 655-662. DOI: 10.1002/cjoc.201400762
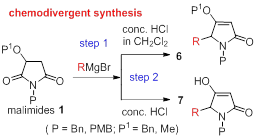
68. Pei-Qiang Huang,* Yu Wang, Kai-Jiong Xiao, and Ying-Hong Huang, A General Method for the Direct Transformation of Common Tertiary Amides into Ketones and Amines by Addition of Grignard Reagents, Tetrahedron 2015, 71, 4248-4254. DOI: 10.1016/j.tet.2015.04.074 三区
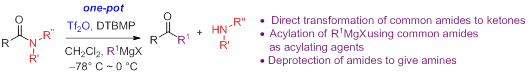
69. Hui Geng, Pei-Qiang Huang*, Versatile and Chemoselective Transformation of Aliphatic and Aromatic Secondary Amides to Nitriles, Tetrahedron 2015, 71, 3795-3801. DOI: 10.1016/j.tet.2015.04.074

70. Jian-Liang Ye,* Yu-Feng Zhang, Yang Liu, Jin-Yuan Zhang, Yuan-Ping Ruan and Pei-Qiang Huang* Studies on the Asymmetric Synthesis of Pandamarilactonines: An Unexpected syn-Selective Vinylogous Mannich Reaction of N-tert-Butanesulfinimines,Org. Chem. Front. 2015, 2, 697-704. DOI: 10.1039/C5QO00098J
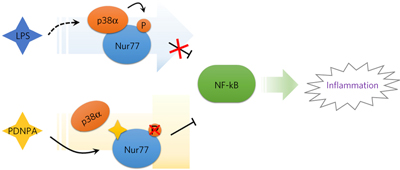
71. Li Li, Yuan Liu, Hang-zi Chen, Feng-wei Li, Jian-feng Wu, Hong-kui Zhang, Jian-ping He, Yong-zhen Xing, Yan Chen, Wei-jia Wang, Xu-yang Tian, An-zhong Li, Qian Zhang, Pei-qiang Huang, Jiahuai Han, Tianwei Lin* & Qiao Wu,* Impeding the interaction between Nur77 and p38 reduces LPS induced inflammation,Nature Chem. Biol. 2015, 11, 339-346.
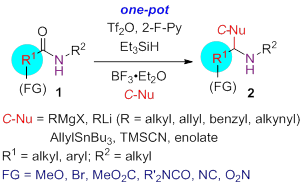
72. Pei-Qiang Huang,* Ying-Hong Huang, Kai-Jiong Xiao, Yu Wang, and Xiao-Er Xia, A General Method for the One-pot Reductive Functionalization of Secondary Amides,J. Org. Chem. 2015, 80, 2861-2868. DOI: 10.1021/jo502929x (highlighted by Organic Chemistry Portal).
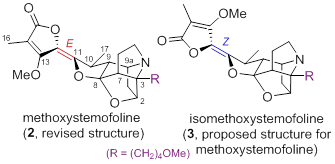
73. Pei-Qiang Huang,* Su-Yu Huang, Long-Hui Gao, Zhong-Yi Mao, Zong Chang, Ai-E Wang, Enantioselective Total Synthesis of (+)-Methoxystemofoline and (+)-Isomethoxystemofoline, Chem. Commun. 2015, 51, 4576-4578. Corrigenda: Chem. Commun. 2016, 52, 4840 – 4840. DOI: 10.1039/C4CC09598G; DOI: 10.1039/C6CC90127A
74. Huang Pei-Qiang,* Geng Hui, Tian Yong-Song, Peng Qiu-Ran, Xiao Kai-Jiong, The First Enantioselective Total Synthesis of (+)-Preussin B and an Improved Synthesis of (+)-Preussin by Step-economical Methods, Sci. China-Chem. 2015, 58, 478-482. DOI: 10.1007/s11426-014-5270-0
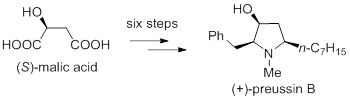
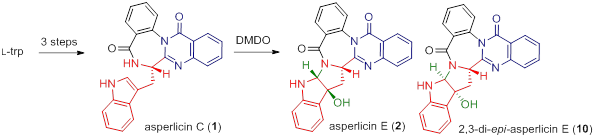
75. Pei-Qiang Huang,* Yu Wang,‡ Shi-Peng Luo,‡ Hui Geng, Yuan-Ping Ruan, and Ai-E Wang, Procedure-Economical Enantioselective Total Syntheses of Asperlicins C and E, Tetrahedron Lett. 2015, 56, 1255-1258. DOI: 10.1016/j.tetlet.2015.01.084
76. Ai-E Wang,* Zong Chang, Wei-Ting Sun, and Pei-Qiang Huang,* General and Chemoselective Bisphosphonylation of Secondary and Tertiary Amides Org. Lett., 2015, 17, 732–735. DOI: 10.1021/acs.orglett.5b00004
77. Pei-Qiang Huang* and Hui Geng,Simple, Versatile, and Chemoselective Reduction of Secondary Amides and Lactams to Amines with Tf2O - NaBH4 or Cp2ZrHCl - NaBH4 System, Org. Chem. Front. 2015, 2, 150-158. DOI: 10.1039/C4QO00317A
78. Xiao Zheng,* Juan Liu, Chen-Xi Ye, Ao Wang, Ai-E Wang, and Pei-Qiang Huang, A SmI2-Mediated Radical Coupling Strategy to Securinega Alkaloids: Total Synthesis of (−)-14,15-Dihydrosecurinine and Formal Total Synthesis of (−)-Securinine J. Org. Chem. 2015, 80, 1034–1041. DOI: 10.1021/jo502522x
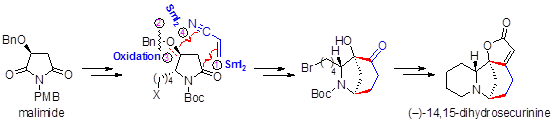
79. Pei-Qiang Huang,* Qi-Wei Lang, Ai-E Wang and Jian-Feng Zheng, Direct Reductive Coupling of Secondary Amides: Chemoselective Formation of Vicinal Diamines and Vicinal Amino Alcohols, Chem. Commun. 2015, 51, 1096-1099. DOI: 10.1039/C4CC08330J
2014
80. Shi-Peng Luo, Qi-Long Peng, Chu-Pei Xu, Ai-E Wang, Pei-Qiang Huang,* Bioinspired Step-Economical, Redox-Economical and Protecting-Group-Free Enantioselective Total Syntheses of (-)-Chaetominine and Analogues, Chin. J. Chem. 2014, 32, 757-770. (the eighth highly cited paper in 2015, see: Chin. J. Chem. 2016, 34, 3-4)
81. Ai-E Wang and Pei-Qiang Huang,* Efficient Asymmetric Syntheses of Alkaloids and Medicinally Relevant Molecules Based on Heterocyclic Chiral Building Blocks, Pure Appl. Chem. 2014, 86, 1227-1235.
82. Pei-Qiang Huang,* Wei Ou, Kai-Jiong Xiao, and Ai-E Wang, Tertiary Amides-based Knoevenagel-type Reactions: A Direct, General, and Chemoselective Approach to Enaminones, Chem. Commun. 2014, 50, 8761-8763. DOI: 10.1039/C4CC03826F
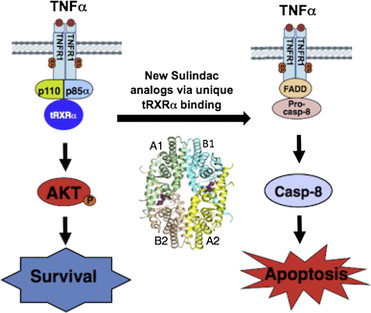
83. Liqun Chen1,2,#, Zhi-Gang Wang3,#, Alexander Aleshin2,#, Fan Chen1,2, Jiebo Chen1,2, Fuquan Jiang1, Gulimiran Alitongbieke2, Zhiping Zeng1, Yue Ma1, Mingfeng Huang1, Hu Zhou1,2, Gregory Cadwell2, Jian-Feng Zheng3, Pei-Qiang Huang3, Robert Liddington2, Xiao-kun Zhang1,2*, and Ying Su1,2* Sulindac-derived RXRa modulators inhibit cancer cell growth by binding to a novel site of RXRa, Chem. & Biol. 2014, 21, 596-607. doi: 10.1016/j.chembiol.2014.02.017 (Cell Press)
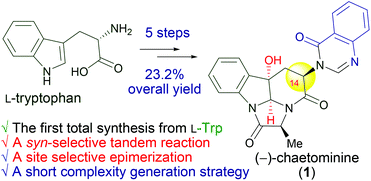
84. Chu-Pei Xu,a Shi-Peng Luo,a Ai-E Wang,a and Pei-Qiang Huang,* Complexity Generation by Chemical Synthesis: Five-Step Synthesis of (-)-Chaetominine from L-Tryptophan and its Biosynthetic Implications, Org. Biomol. Chem. 2014, 12, 2859-2863. DOI: 10.1039/C4OB00314D.
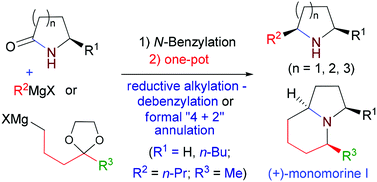
85. Hui-Qiong Deng, Xiang-Yang Qian, Yu-Xiu Li, Jian-Feng Zheng, Lin-Feng Xie,* and Pei-Qiang Huang,* A Versatile Two-step Method for the Reductive Alkylation and Formal [4 + 2] Annulation of Secondary Lactams: Step Economical Syntheses of the ant venom Alkaloids cis-2-Butyl-5-propylpyrrolidine and (+)-Monomorine I,Org. Chem. Front. 2014, 1, 258-266. DOI: 10.1039/C3QO00065F.
86. X.-G. Wang, A. E Wang, and P.-Q. Huang,* A Concise Formal Stereoselective Total Synthesis of (-)-Swainsonine, Chinese Chem. Lett. 2014, 23, 193-196. DOI: 10.1016/j.cclet.2013.12.003兰州
87. Zhong-Yi Mao, Su-Yu Huang, Long-Hui Gao, Ai-E Wang, and Pei-Qiang Huang,* A Novel and Versatile Method for the Enantioselective Syntheses of Tropane Alkaloids, Science China-Chem. 2014, 57, 252-264. doi: 10.1007/s11426-013-4998-2.
88. Qi-Long Peng, Shi-Peng Luo, Xiao-Er Xia, Liang-Xian Liu, and Pei-Qiang Huang,* The Four-Step Total Synthesis of (-)-Chaetominine, Chem. Commun. 2014, 50, 1986-1988. (Top 10% of the highly cited authors in the RSC’s General Chemistry portfolio of journals) DOI: 10.1039/C3CC48833K.
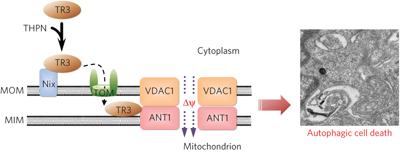
89. Wei-jia Wang, Yuan Wang, Hang-zi Chen, Yong-zhen Xing, Feng-wei Li, Qian Zhang, Bo Zhou, Hong-kui Zhang, Jie Zhang, Xue-li Bian, Li Li, Yuan Liu, Bi-xing Zhao, Yan Chen, Rong Wu, An-zhong Li, Lu-ming Yao, Ping Chen, Yi Zhang, Xu-yang Tian, Friedrich Beermann, Mian Wu, Jiahuai Han, Pei-qiang Huang, Tianwei Lin* & Qiao Wu,* Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway, Nature Chem. Biol. 2014, 10, 133-140. doi: 10.1038/nchembio.1406.
2013
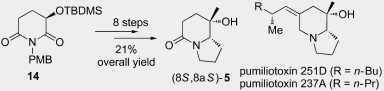
90. Jie Zhang, Hong-Kui Zhang*, and Pei-Qiang Huang,* Towards Stereochemical Control: A Short Formal Enantioselective Total Synthesis of Pumiliotoxins 251 D and 237 A, Beil. J. Org. Chem. 2013, 9, 2358–2366. (Invited for the Thematic Series: Natural products in synthesis and biosynthesis)
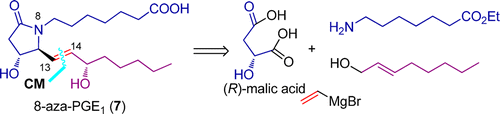
91. Xiao-Gang Wang, Ai-E Wang, Yi Hao, Yuan-Ping Ruan, and Pei-Qiang Huang,* The Modular Enantioselective Synthesis of 8-Aza-prostaglandin E1, J. Org. Chem. 2013, 78, 9488-9493. doi.org/10.1021/jo401412g.
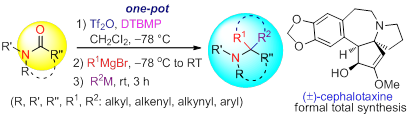
92. Kai-Jiong Xiao, Jie-Min Luo, Xiao-Er Xia, Yu Wang, and Pei-Qiang Huang*, General One-pot Reductive gem-Bis-alkylation of Tertiary Lactams/Amides: Rapid Construction of 1-Azaspirocycles and Formal Total Synthesis of (-)-Cephalotaxine, Chem. Eur. J. 2013, 19, 13075-13086. DOI: 10.1002/chem.201302096 (highlighted by ChemPubSoc Eur. on the ChemistryViews website)
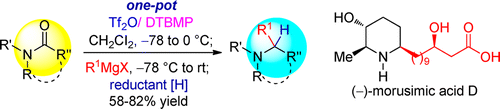
93. Kai-Jiong Xiao, Yu Wang, Ying-Hong Huang, Xiao-Gang Wang, Jin-Cheng Liao, Pei-Qiang Huang,* A Direct and General Method for the Reductive Alkylation of Tertiary Lactams/Amides: Application to the Step-Economical Synthesis of Bioactive Alkaloid (–)-Morusimic acid, J. Org. Chem. 2013, 78, 8305-8311. DOI: 10.1021/jo4007656.
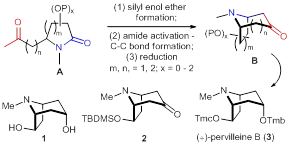
94. Su-Yu Huang, Zong Chang, Shi-Chuan Tuo, Long-Hui Gao, Ai-E Wang, and Pei-Qiang Huang,* Versatile Construction of Functionalized Tropane Ring Systems Based on Lactam Activation: Enantioselective Synthesis of (+)-Pervilleine B, Chem. Commun. 2013, 49, 7088-7090. DOI:10.1039/C3CC43665A.
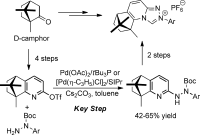
95. Liang-An Chen, Chuan-Fei Wang, Ming-Gui Lin, Jin-Long Zhang, Pei-Qiang Huang, and Ai-E Wang,*Design and Synthesis of Camphor-derived Chiral 1,2,4-Triazolo [4,3-a]-tetrahydroquinoline N-Heterocyclic Carbene Precursors by Pd-Catalyzed Coupling Reactions of Aryl Hydrazides with a Pyridyl Triflate Derivative, Asian J. Org. Chem. 2013, 2, 294 – 298.
96. Jin-Long Zhang, Liang-An Chen, Rui-Bin Xu, Chuan-Fei Wang, Yuan-Ping Ruan, Ai-E Wang,* Pei-Qiang Huang, Chiral imidazo[1,5-a]tetrahydroquinoline N-heterocyclic carbenes and their copper complexes for asymmetric catalysis,Tetrahedron: Asymmetry 2013, 24, 492-498.
97. Lian-Dong Guo, Pan Liang, Jian-Feng Zheng, and Pei-Qiang Huang,* A Concise and Divergent Approach to Hydroxylated Piperidine Alkaloids and Azasugar Lactams, Eur. J. Org. Chem. 2013, 2230-2236.
98. Xiao Zheng,* Xi-Jie Dai, Hong-Qiu Yuan, Chen-Xi Ye, Jie Ma and Pei-Qiang Huang,Umpolung of Hemiaminals: Titanocene-Catalyzed Dehydroxylative Radical Coupling Reactions with Activated Alkenes, Angew. Chem. Int. Ed. 2013, 52, 3494-3498. Angew. Chem. 2013, 125, 3578-3582; DOI: 10.1002/anie.201210088. (Highlighted by Organic Chemistry Portal and Chin. J. Org. Chem.).
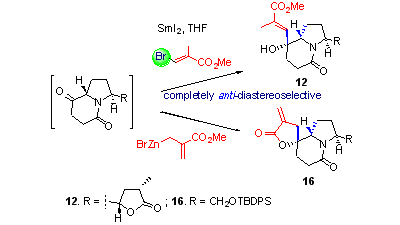
99. Kong-Zhen Hu, Jie Ma, Shi Qiu, Xiao Zheng, and Pei-Qiang Huang,* SmI2-Mediated Intermolecular Coupling of Lactam N-a-Radicals with Activated Alkenes: Asymmetric Synthesis of 11-Hydroxylated Analogues of the Lead Compounds CP-734432 and PF-04475270, J. Org. Chem. 2013, 78, 1790-1801. doi.org/10.1021/jo301277n. (Special issue dedicated to Professor H. E. Zimmerman) Publication Date (Web): August 1, 2012 (Article). (highlighted by Organic Chemistry Portal).
100. Zhi-Gang Wang c, Liqun Chena,b, Jiebo Chena, Jian-Feng Zhengc, Weiwei Gaob, Hu Zhoua,b, Xiao-kun Zhanga,b, Pei-Qiang Huangc*, Ying Sua*,Synthesis and SAR study of modulators inhibiting tRXRα-dependent AKT activation, Eur. J. Med. Chem. 2013, 62, 632–648.
101. Shi-Chuan Tuo, Xue-Kui,Liu, Pei-Qiang Huang, * Towards Stereochemical Control: Two Approaches for the Highly anti-Diastereoselective Construction of the Spirolactone Moieties of Some Stemona Alkaloids, Chinese J. Chem. 2013, 31, 55-62. (special issue in memory of the late Prof. Wei-Shan Zhou) Article first published online: 11 DEC 2012 | DOI: 10.1002/cjoc.20120090
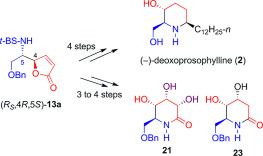
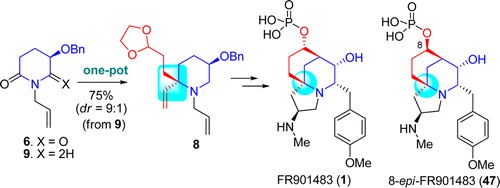
102. Hao-Hua Huo, Xiao-Er Xia, Hong-Kui Zhang,* Pei-Qiang Huang,* Enantioselective Total Syntheses of (-)-FR901483 and (+)-8-epi-FR901483, J. Org. Chem. 2013, 78, 455-465. DOI: http://dx.doi.org/10.1021/jo302362b Publication Date (Web): December 5, 2012 (Article)
103. Xue-Kui Liu, Jian-Liang Ye, Yuan-Ping Ruan, Yu-Xiu Li and Pei-Qiang Huang,* The Total Synthesis of (-)-Sessilifoliamide J, J. Org. Chem. 2013, 78, 35-41. DOI: 10.1021/jo3014484. (Special issue dedicated to Professor R. E. Ireland).
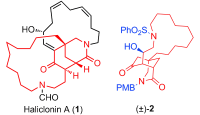
104. Shi-Peng Luo, Lian-Dong Guo, Long-Hui Gao, Shuang Li, Pei-Qiang Huang*,Toward the Total Synthesis of Haliclonin A: Construction of a Tricyclic Substructure,Chem. Eur. J. 2013, 19, 87-91. Doi: 10.1002/chem.201203203.
2012
105. Yu-Huang Wang, Wei Ou, Linfeng Xie, Jian-Liang Ye, and Pei-Qiang Huang*, Towards Reaction Control: cis-Diastereoselective Reductive Dehydroxylation of 5-Alkyl-4-benzyloxy-5-hydroxy-2-pyrrolidinones,Asian J. Org. Chem. 2012, 1, 359-365. DOI: 10.1002/ajoc.201200113
106. Jian-Feng Zheng, Hong-Qiao Lan, Rui-Feng Yang, Qi-Long Peng, Zhen-Hua Xiao, Shi-Chuan Tuo, Kong-Zhen Hu, Yong-Gang Xiang, Zhen Wei, Zhen Zhang, Pei-Qiang Huang,* Asymmetric Syntheses of the Sex Pheromones of Pine Sawflies, Their Homologues and Stereoisomers,Helv. Chim. Acta 2012, 95, 1799-1808. doi: 10.1002/hlca.201200341 (invited to the special issue in honor of Prof. Dieter Seebach on occasion of his 75th Anniversary)
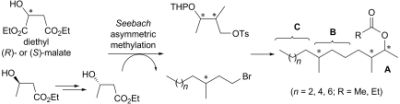
107. Yan-yan Zhan1,4, Yan Chen, Qian Zhang1,4, Jia-jia Zhuang2, Min Tian1, Hang-zi Chen1, Lian-ru Zhang1, Hong-kui Zhang2, Jian-ping He1, Wei-jia Wang1, Rong Wu1, Yuan Wang1, Chunfang Shi1, Kai Yang1, An-zhong Li1, Yong-zhen Xin1, Terytty Yang Li1, James Y Yang1, Zhong-hui Zheng1, Chun-dong Yu1, Sheng-Cai Lin1, Chawnshang Chang3, Pei-qiang Huang2, Tianwei Lin1* & Qiao Wu1*The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK, Nature Chem. Biol. 2012, 8, 897–904. doi:10.1038/nchembio.1069.
108. 肖开炯, 黄应红, 黄培强, 仲酰胺经酰胺活化直接合成酮的普适性方法, 化学学报 2012, 70, 1917-1922. General Direct Transformation of Secondary Amides to Ketones via Amide Activation, Xiao, K.-J.; Huang, Y.-H.; Huang, P.-Q. Acta Chim. Sinica 2012, 70, 1917-1922 (invited)
109. Hao-Hua Huo, Hong-Kui Zhang, Xiao-Er Xia, Pei-Qiang Huang,* A Formal Enantioselective Total Synthesis of FR901483,Org. Lett. 2012, 14, 4834-4837. DOI: 10.1021/ol302165d.
110. Xi-Jie Dai and Pei-Qiang Huang,*A Short and Flexible Synthetic Approach to the Naturally Occurring Racemic Neoclausenamide and its Analogs, Chinese J. Chem. 2012, 30, 1953-1956. (Invited to the special issue on occasion of the 80th Anniversary of Chinese Chemical Society)
111. Kai-Jiong Xiao, Ai-E Wang, Ying-Hong Huang, and Pei-Qiang Huang,* Versatile and Direct Transformation of Secondary Amides into Ketones via Organocerium Reagents-Based Deaminative Alkylation, Asian J. Org. Chem. 2012, 1, 130-132. Article first published online: 19 SEP 2012 | DOI: 10.1002/ajoc.201200066 (Invited, cover picture of issue 2). Featured by in the news section of ChemPubSoc Europe's Chemistry Views magazine:
http://www.chemistryviews.org/details/ezine/2525331/Ceri-ously_Simple.html
The most cited communication published in AJOC during 2012-2014.
112. Kai-Jiong Xiao, Ai-E Wang, and Pei-Qiang Huang,* Direct Transformation of Secondary Amides into Secondary Amines: Triflic Anhydride Activated Reductive Alkylation, Angew. Chem., Int. Ed. 2012, 51, 8314-8317; Angew. Chem. 2012, 124, 8439-8442. DOI: 10.1002/anie.201204098. (highlighted by Advances in Engineering)
113. Yu-Huang Wang, Jian-Liang Ye, Ai-E Wang, and Pei-Qiang Huang,* Reductive Hydroxyalkylation/ Alkylation of Amines with Lactones/ Esters, Org. Biomol. Chem. 2012, 10, 6504-6511. DOI: 10.1039/c2ob25901j
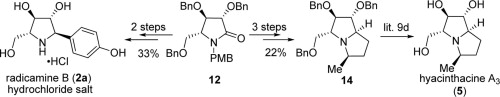
114. Jin-Cheng Liao, Kai-Jiong Xiao, Xiao Zheng, and Pei-Qiang Huang,*A Concise and Divergent Approach to Radicamine B and Hyacinthacine A3 Based on a Step-economic Transformation, Tetrahedron 2012, 68, 5297-5302. (invited to Shi special issue).
115. Hong-Kui Zhang,* Shou-Qiang Xu, Jia-Jia Zhuang, Jian-Liang Ye, Pei-Qiang Huang, A flexible enantioselective approach to 3,4-dihydroxyprolinol derivatives by SmI2-mediated reductive coupling of chiral nitrone with ketones/aldehydes, Tetrahedron 2012, 68, 6656-6664.
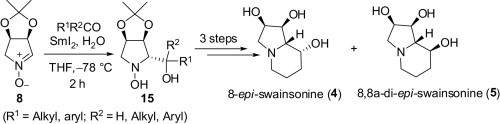
116. Chu-Pei Xu, Pei-Qiang Huang*, and Sandrine Py,* SmI2-Mediated Coupling of Nitrones and tert-Butanesulfinyl Imines with Allenoates: Synthesis of b-Methylenyl-g-lactams and Tetramic Acids, Org. Lett. 2012, 14, 2034-2037. (highlighted by Synfacts).
117. Jie Chen, Ai-E Wang,* Hao-Hua Huo, Pei-Qiang Huang,* Recent progress on the total synthesis of natural products in China, Science China-Chem. 2012, 55, 1175-1212.
118. Gui-Yang Chen, Jian-Liang Ye, Hui-Ying Huang, Yuan-Ping Ruan, Ai-E Wang, and Pei-Qiang Huang,* Divergent Enantioselective Synthesis of Rigidiusculamide B and the Proposed Structure of Rigidiusculamide A: Revision of the Relative Stereochemistry of Rigidiusculamide A, Chem. Asian J. 2012, 7, 504-518. 10.1002/asia.201100809.(frontispiece cover picture)
119. Jia-Jia Zhuang, Jian-Liang Ye, Hong-Kui Zhang,* Pei-Qiang Huang, An Unexpected High erythro-Selection in the Grignard Reaction with an N,O-Acetal: A Concise Asymmetric Synthesis of Indolizidine Alkaloid (-)-2-epi-Lentiginosine, Tetrahedron 2012, 68, 1750-1755.
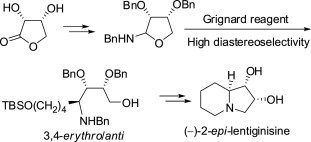
120. Xue-Kui Liu, Xiao Zheng, Yuan-Ping Ruan, Jie Ma, and Pei-Qiang Huang,* One-Pot Reductive Coupling of N-Acylcarbamates with Activated Alkenes: Application to the Asymmetric Synthesis of Pyrrolo[1,2-a]azepin-5-one Ring System and (-)-Xenovenine, Org. Biomol. Chem. 2012, 10, 1275-1284.
121. Zhi-Gang Wang, Jian-Feng Zheng,* Pei-Qiang Huang, Asymmetric Synthesis of Both Enantiomers of Disparlure, Chinese J. Chem. 2012, 30, 23-28. (Cover article)
2011
122. Bi-Shuang Chen, Long-He Yang, Jian-Liang Ye, Tao Huang, Yuan-Ping Ruan, Jin Fu* and Pei-Qiang Huang,* Diastereoselective Synthesis and Bioactivity of Long-chain anti-2-Amino-3-alkanols, Eur. J. Med. Chem. 2011, 46, 5480-5486.
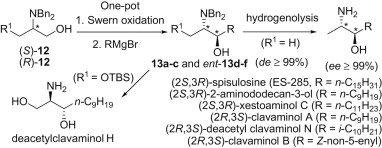
123. Shi-Chuan Tuo, Jian-Liang Ye, Ai-E Wang, Su-Yu Huang, Pei-Qiang Huang,* Concise Asymmetric Total Synthesis of 9-epi-Sessilifoliamide J, Org. Lett. 2011, 13, 5270-5273.
124. Shu-Tang Ruan, Jie-Min Luo, Yu Du, and Pei-Qiang Huang,*Asymmetric Vinylogous Mannich Reactions: A Versatile Approach to Functionalized Heterocycles, Org. Lett. 2011, 13, 4938-4941.
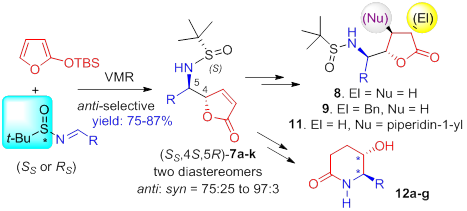
125. Xue-Kui Liu, Shi Qiu, Yong-Gang Xiang, Yuan-Ping Ruan, Xiao Zheng,* and Pei-Qiang Huang,* SmI2-Mediated Radical Cross-Couplings of a-Hydroxylated Aza-Hemiacetals and N,S-Acetals with a,b-Unsaturated Compounds: Asymmetric Synthesis of (+)-Hyacinthacine A2, (-)-Uniflorine A, and (+)-7-epi-Casuarine, J. Org. Chem. 2011, 76, 4952-4963. (highlighted by Organic Chemistry Portal).
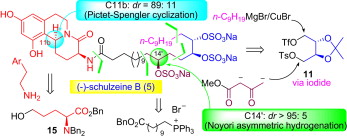
126. Chao Yang, Yu-Hui Bao, Pan Liang, Jian-Liang Ye, Ai-E Wang, and Pei-Qiang Huang,* A Concise and Highly Diastereoselective Synthesis of the Marine Alkaloid (-)-Schulzeine B via Chiron Approach, Tetrahedron 2011, 67, 6281-6288.
127. Hong-Kui Zhang,* Xin Li, Huang Huang and Pei-Qiang Huang,* Asymmetric Syntheses of (8R,8aS)- and (8R,8aR)-8-Hydroxy-5-indolizidinones: Two Promising Oxygenated Indolizidine Building Blocks, Scientia Sinica Chimica. 2011, 41, 732-740 (in Chinese); Science China-Chem. 2011, 54, 737-744. (Cover article)
128. Bo Teng, Jian-Feng Zheng,* Hui-Ying Huang and Pei-Qiang Huang,* Enantioselective Synthesis of Glutarimide Alkaloids Cordiarimides A, B, Crotonimides A, B, and Julocrotine, Chinese J. Chem. 2011, 29, 1312-1318.
129 Zhen-Hua Xiao, Liang-Xian Liu,* Cong Liu, Pei-Qiang Huang,* A Flexible Approach to Protected (4S,5S)-5-Alkyl-1-benzyl-4-benzyloxy-2-pyrrolidinones, Synth. Commun. 2011, 41, 2036-2043.
130. Jin-Li Zheng, Hui Liu, Yu-Feng Zhang, Wei Zhao, Jin-Shuan Tong, Yuan-Ping Ruan,* and Pei-Qiang Huang,* A study on the racemization step in the synthesis of pyrrolidinols via cyclic a-hydroxyimides, Tetrahedron: Asymmetry 2011, 22, 257-263.
131. Geng-Jie Lin, Xiao Zheng and Pei-Qiang Huang,* A New Method for the Construction of the Hydroxylated Tropane Skeleton: Enantioselective Synthesis of (-)-Bao Gong Teng A, Chem. Commun. 2011, 47, 1545-1547. DOI: 10.1039/C0CC04371K
132. Hong-Qiao Lan, Jian-Liang Ye, Ai-E Wang, Pei-Qiang Huang,* Asymmetric Synthesis of 5-Alkyl Tetramates and the Asymmetric Total Synthesis of Marine Natural Product Belamide A Chem. Eu r. J. 2011, 17, 958-968.
133. Yu Du, Hui-Ying Huang, Hui Liu, Yuan-Ping Ruan, and Pei-Qiang Huang,* Studies towards the Asymmetric Synthesis of the Pentacyclic Indole Alkaloid Arboflorine: Asymmetric Synthesis of a Key Intermediate, Synlett 2011, 565-569.
2010
134. ZHENG Xiao, ZHU Wen-Fang, HUANG Pei-Qiang,* Concise synthesis of two advanced intermediates for the asymmetric synthesis of polyhydroxylated indolizidine alkaloids, Science China: Chem. 2010, 53, 1914-1920. (Cover article)
135. Hong-Kui Zhang,* Zhi-Jie Lin, Huang Huang, Hao-Hua Huo, Yan-Ju Huang, Jian-Liang Ye, Pei-Qiang Huang,* On the Enantioselective Synthesis of the TAN1251C of Diazatricyclic Core via Iodoaminocyclization Reaction, Chin. J. Chem. 2010, 28, 1717-1724.
136. Kai-Jiong Xiao, Yu Wang, Ke-Yin Ye, and Pei-Qiang Huang,* Versatile One-pot Reductive Alkylation of Lactams/Amides via Amide Activation: Application to the Concise Syntheses of Bioactive Alkaloids (±)-Bgugaine, (±)-Coniine, (+)-Preussin, and (–)-Cassine, Chem. Eur. J. 2010, 16, 12792-12796.
137. Chu-Pei Xu, Zhen-Hua Xiao, Bi-Qin Zhuo, Yu-Huang Wang and Pei-Qiang Huang,* Efficient and chemoselective alkylation of amines/amino acids using alcohols as alkylating reagents under mild conditions, Chem. Commun. 2010, 46, 7834-7836. (highlighted by Organic Chemistry Portal)
138. Rui-Feng Yang, Pei-Qiang Huang,* Studies towards an Enantioselective Total Synthesis of Sarain A: A Concise Asymmetric Construction of the Diazatricyclic Core, Chem. Eur. J. 2010, 16, 10319-10322.
139. Hu Zhou, Wen Liu, Ying Su, Zhen Wei, Jie Liu, Siva Kumar Kolluri, Hua Wu, Yu Cao, Jiebo Chen,Yin Wu, Tingdong Yan, Xihua Cao, Weiwei Gao, Andrei Molotkov, Fuquan Jiang, Wen-Gang Li, Bingzhen Lin, Hai-Ping Zhang, Jinghua Yu, Shi-Peng Luo, Jin-Zhang Zeng, Gregg Duester, Pei-Qiang Huang, and Xiao-Kun Zhang,* NSAID Sulindac and Its Analog Bind RXRa and Inhibit RXRa-Dependent AKT Signaling, Cancer Cell 2010, 17, 560-573 (featured article).
140. Jing-jing Liu, Hui-ni Zeng, Lian-ru Zhang, Yan-yan Zhan, Yan Chen, Yuan Wang, Juan Wang, Shao-hua Xiang, Wen-jun Liu, Wei-jia Wang, Hang-zi Chen, Yue-mao Shen, Wen-jin Su, Pei-qiang Huang, Hong-kui Zhang,* and Qiao Wu,* A Unique Pharmacophore for Activation of the Nuclear Orphan Receptor Nur77 In vivo and In vitro Cancer Res. 2010, 70, 3628-3637.
141. Fu, Rui, Ye, Jian-Liang,* Dai, Xi-Jie, Ruan, Yuan-Ping, Huang, Pei-Qiang,* Asymmetric Synthesis of the Cytotoxic Marine Natural Product (+)-Awajanomycin and Its C-11 Epimer, J. Org. Chem. 2010, 75, 4230-4243.
142. Yu Du, Jian-Feng Zheng, Li-Jiao Jiang, and Pei-Qiang Huang,* A Concise, Protection-Free and Divergent Approach for the Enantioselective Syntheses of Two Pheromonal Epoxide Components of the Fall Webworm Moth and Other Species, J. Org. Chem. 2010, 75, 4619-4622.
143. Hong-Qiao Lan, Yuan-Ping Ruan and Pei-Qiang Huang,* The first enantioselective synthesis of cytotoxic marine natural product palau’imide and assignment of its C-20 stereochemistry, Chem. Commun. 2010, 46, 5319-5321.
144. Gang Liu, Tian-Jun Wu, Yuan-Ping Ruan, Pei-Qiang Huang,* A Flexible Approach to 2-Hydroxyalkyl 3-hydroxy-2-pyrrolidinone Derivatives: Concise Asymmetric Syntheses of (+)-1-Epi-Castanospermine and (+)-Castanospermine, and 7-Deoxy-6-epi-castanospermine, Chem. Eur. J. 2010, 16, 5755-5768.
145. Xiao, K.-J.; Luo, J.-M.; Ye, K.-Y.; Wang, Y.; Huang, P. –Q.* Direct, One-pot Sequential Reductive Alkylation of Lactams/Amides with Grignard and Organolithium Reagents via Lactam/Amide Activation, Angew. Chem., Int. Ed. 2010, 49, 3037-3040; Angew. Chem. 2010, 122, 3101–3104 (highlighted by Nature Chem. and Synfacts). 方法2011年被E. M. Carreira小组采用。
146. Wen-Jun Liu, Jian-Liang Ye,* and Pei-Qiang Huang,* The Asymmetric Syntheses of Hyacinthacines A2 and A3 by a Flexible Approach and Confirmation of the Proposed Structure for Hyacinthacine A3, Org. Biomol. Chem. 2010, 8, 2085-2091.
147. Shao-Hua Xiang, Jian Xu, Hong-Qiu Yuan, and Pei-Qiang Huang,* Amide Activation by Tf2O: Reduction of Amides to Amines by NaBH4 under Mild Conditions, Synlett 2010, 1829-1832. (highlighted two times by Organic Chemistry Portal)
148. Shao-Feng Wu, Yuan-Ping Ruan, Xiao Zheng, and Pei-Qiang Huang,* Samarium Diiodide-mediated Reductive Couplings of Chiral Nitrones with Aldehydes/Ketones/ and Acyl Chlorides, Tetrahedron 2010, 66, 1653-1660.
149. Zhao-Bao Ye, Jie Chen, Wei-Hua Meng, Pei-Qiang Huang,* N-Benzyloxy-malimide for a Flexible Access to 5-Alkyl-3-pyrrolin-2-ones: Asymmetric Synthesis of the Mixed Imide Portion of Microcolin B, Tetrahedron: Asymmetry 2010, 21, 895-902.
150. Li-Jiao Jiang, Bo Teng, Jian-Feng Zheng, Jian-Liang Ye, and Pei-Qiang Huang,* Flexible Template-controlled Syntheses of N-a-Alkyllactam Derivatives via Action of Two Complementary Lewis Acids, Tetrahedron 2010, 66, 172-175.
2009
151. Shao-Hua Xiang, Hong-Qiu Yuan, Pei-Qiang Huang,* A Versatile Approach to cis-5-Substituted 4-hydroxy-2-pyrrolidinones: Asymmetric Synthesis of Angiogenesis Inhibitor Streptopyrrolidine. Tetrahedron: Asymmetry 2009, 20, 2021-2026.
152. Jie Chen, Pei-Qiang Huang,* and Yves Queneau,* Enantioselective Synthesis of the Broadly Acting Feeding Deterrent (R)-Ypaoamide, J. Org. Chem. 2009, 74 (19), 7457–7463.
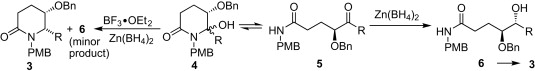
153. .Geng-Jie Lin, Pei-Qiang Huang,* A Concise and Fully Selective Synthesis of the Ant Venom Alkaloid (3S,5R,8S,9S)-3-Butyl-5-propyl-8-hydroxyindolizidine, Org. Biomol. Chem. 2009, 7, 4491-4495.
154. Xiang, Y.-G.; Wang, X.-W.; Zheng, X.;* Ruan, Y.-P.; Huang, P. -Q.* One-pot Cross-coupling of N-Acyl N,O-Acetals with a,b-Unsaturated Compounds, Chem. Commun. 2009, 7045-7047.
155. Zheng, Xiao,* Chen, Guo, Ruan Yuan-Ping,* Huang, Pei-Qiang, Generation and a-Hydroxyalkylation of a Novel 3-Piperidinol N-a-Carbanion Intermediate, Sci. China Ser. B-Chem. 2009, 52 (10), 1631-1638; Scientia Sinica Chim. 2008, 39, 1175-1183.中文版:3-羟基哌啶氮α-碳负离子的形成及α-羟烷化反应,中国科学 B 辑:化学,2008, 39, 1175- 1183.
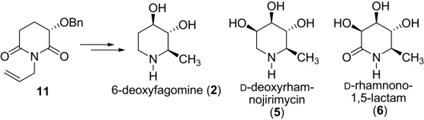
156. Rui Fu, Yu Du, Zhao-Ying Li, Wei-Xuan Xu, and Pei-Qiang Huang,* Asymmetric Syntheses of 6-Deoxyfagomin, D-Deoxyrhamnojirimycin, and D-Rhamnono-1,5-lactam Tetrahedron 2009, 65, 9765-9771.
157. Fu, R.; Chen, J.; Guo, L.-C.; Ruan, Y.-P.; Huang, P.-Q.* Asymmetric Total Synthesis of (-)-Awajanomycin, Org. Lett. 2009, 11, 5242-5245.
158. Shao-Feng Wu, Xiao Zheng,* Yuan-Ping Ruan, and Pei-Qiang Huang,* A New Approach to 3-Hydroxyprolinol Derivatives by Samarium Diiodide-mediated Reductive Coupling of Chiral Nitrone with Carbonyl Compounds, Org. Biomol. Chem. 2009, 7, 2967-2975.
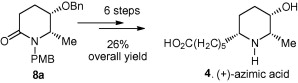
159. Kai-Jiong Xiao, Liang-Xian Liu,* and Pei-Qiang Huang,* An Enantioselective Synthesis of (+)-Azimic Acid, Tetrahedron: Asymmetry, 2009, 20, 1181-1184.
160. Luo, J.-M.; Dai, C.-F.; S.-Y. Lin; Huang, P.-Q.* Asymmetric Syntheses and Wnt Signal Inhibitory Activity of Melleumin A and Four Analogues of Melleumins A, B Chem. Asian J. 2009, 4, 328 – 335.
161. Liang-Xian Liu,* Kai-Jiong Xiao, and Pei-Qiang Huang,* Chemo- and Diastereo-selective Control for A Flexible Approach to (5S,6S)-6-Alkyl-5-benzyloxy-2-piperidinones, Tetrahedron 2009, 52, 3834-3841.
162. Li-Jiao Jiang, Hong-Qiao Lan, Jian-Feng Zheng,* Jian-Liang Ye, and Pei-Qiang Huang,* A Flexible Approach to Methyl (5S)-5-Alkyltetramate Derivatives, Synlett 2009, 297-301.
163. Wen Chen, Xiao Zheng, Yuan Ping Ruan, and Pei Qiang Huang,* Facile Syntheses of Three Ahp-type Building Blocks with Complementary Reactivity, Heterocycles 2009, 79, 681-693.
164. Z. Wei, H.-Q. Lan, J.-F. Zheng,* and P.-Q. Huang,* “A New and Concise Approach to (R)-a-Lipoic Acid”, Synth. Comm. 2009, 39 (4), 691-701;
2008
165. Chen, C. Y.; Zhou, Z. H,;* Chen, H. B.; Huang, P. Q.; Tsai, K. R.; Chow, Y. L. Formations of mixed-valence oxovanadium (V,IV) citrates and homocitrate with N-heterocycle chelated ligand, Inorg. Chem. 2008, 47, 8714-8720..
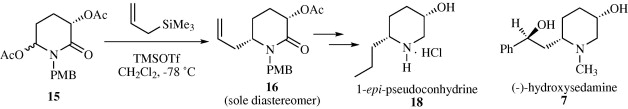
166. Pei-Qiang Huang,* Jie Meng, Gang Liu, Chen-Guo Feng, and Jie Chen, “Asymmetric Syntheses of (-)-epi-Pseudoconhydrine and (-)-5-Hydroxysedamine Based on cis-Diastereoselective 1,4-Asymmetric Induction”, Tetrahedron: Asymmetry, 2008, 19 (11), 1297-1303.
167. Liang-Xian Liu,* Qi-Long Peng, Pei-Qiang Huang,* A new approach for the asymmetric synthesis of (2S,3S)-3-hydroxypipecolic acid, Tetrahedron: Asymmetry, 2008, 19 (10), 1200-1203.
168. De-Sheng Yu, Wei-Xuan Xu, Liang-Xian Liu, and Pei-Qiang Huang,* First Asymmetric Synthesis of Piperidine Alkaloid (-)-Morusimic acid D, Synlett 2008, 1189-1192.
169. Geng-Jie Lin, Shi-Peng Luo, Xiao Zheng, Jian-Liang Ye, and Pei-Qiang Huang,* Enantiodivergent Synthesis of trans-3,4-Disubstituted Succinimides by SmI2-Mediated Reformatsky Type Reaction, Tetrahedron Lett. 2008, 49 (25), 4007-4010.
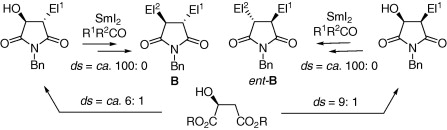
170. Tian-Jun Wu, Pei-Qiang Huang,* A Concise Approach to (+)-1-Epi-castanospermine, Tetrahedron Lett. 2008, 49 (2), 383-386.
171. Xiang Zhou, Jian-Liang Ye and Pei-Qiang Huang,* "A Versatile Approach to (4S,5R)-4-Benzyloxy-5-([a-hydroxyalkyl)-2-pyrrolidinones:Experimental Evidences to the Computational Predictions", Comptes Rendus de l'Académie des Sciences – CHIMIE (Comptes Rendus–CHIMIE), 2008, 11, 5-18.
172. Jian-Feng Zheng, Wen Chen, Jian-Liang Ye, Su-Yu Huang and Pei-Qiang Huang,* “A divergent asymmetric approach to aza-spiropyran derivative and (1S,8aR)-1-hydroxyindolizidine”, Beil. J. Org. Chem. 2007, 3:41, 1-5.
173. Xiang Zhou, Wen-Jun Liu, Jian-Liang Ye and Pei-Qiang Huang,* "Complementary Stereocontrolled Approaches to 2-Pyrrolidinones Bearing Vicinal Amino Diol Subunit with Three Continuous Chiral Centers: A Formal Asymmetric Synthesis of (-)-Detoxinine", J. Org. Chem. 2007, 72 (23), 8904-8909.
174. Chao-Feng Dai, Fang Cheng, Hai-Chao Xu, Yuan-Ping Ruan,and Pei-Qiang Huang,* "Diversity-Oriented Asymmetric Synthesis of Hapalosin: Construction of Three Small C-9/ C-4/ C-3-Modified Hapalosin Analogues Libraries" J. Comb. Chem. 2007, 9 (3), 386-394.
175. Jian-Liang Ye, Pei-Qiang Huang,* Xin Lu,* Mechanism for the Regioselective Asymmetric Addition of Grignard, Reagents to Malimides: A Computational Exploration, J. Org. Chem. 2007, 72 (1), 35-42.
176. X. Zhou, W.-J. Liu; J.-L. Ye, and P.-Q. Huang,* A Versatile Approach to Pyrrolidine Azasugars and Homoazasugars Based on a Highly Diastereoselective Reductive Benzyloxymethylation of Protected Tartarimide, Tetrahedron 2007, 50 (27), 6346-6357. (Invited)
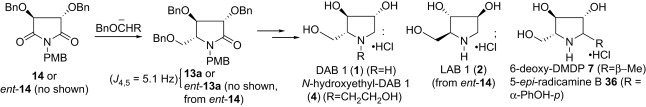
177. Pei-Qiang Huang,* Guo Chen, and Xiao Zheng, A New Synthesis of alkaloid (S)-3-Hydroxypiperidin-2-one and Its O-TBS Protected Derivative, J. Heterocyclic Chem. 2007, 44 (2): 499-501.
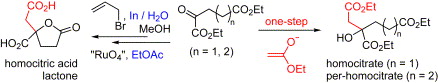
178.Hong-Bin Chen, Ling-Yan Chen, Pei-Qiang Huang,* Hong-Kui, Zhang, Zhao-Hui Zhou, and Khi-Rui Tsai, Expeditious Biomimetically-Inspired Approaches to Racemic Homocitric Acid Lactone and Per-homocitrate, Tetrahedron 2007, 63 (10), 2148-2152.
179. Pei-Qiang Huang, Asymmetric Synthesis of Hydroxylated Pyrrolidines, Piperidines and Related Bioactive Compounds: from N-Acyliminium Chemistry to N-a-Carbanion Chemistry,Synlett 2006, 1133-1147 (Account).
180. Chen-Guo Feng, Jie Chen, Jian-Liang Ye, Yuan-Ping Ruan, Xiao Zheng and Pei-Qiang Huang,* Syntheses of Enantio-enriched Chiral Building Blocks from L-Glutamic Acid, Tetrahedron 2006, 62 (31), 7459-7465.
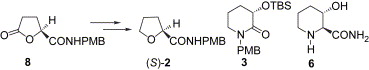
181. Liang-Xian Liu, Pei-Qiang Huang,* A Formal SN2 Reaction of 2-Substituted 3-Piperidinol Mesylate with Retention of Configuration:Application to the Asymmetric Synthesis of (2R,3S)-CP-99,994,Tetrahedron: Asymmetry 2006, 16 (23), 3265-3272. Correction: Tetrahedron: Asymmetry, 2007, 18, 155
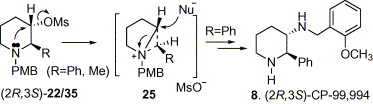
182. Xiang Zhou and Pei-Qiang Huang,* A Versatile Approach to Protected (4S,5R)-4-Hydroxy-5-(a-hydroxyalkyl)-2-pyrrolidinones, Synlett 2006, 1235-1239.
183. Pei-Qiang Huang,* Zheng-Qing Guo, and Yuan-Ping Ruan, A Versatile Approach for the Asymmetric Syntheses of (1R,9aR)-Epiquinamide and (1R,9aR)-Homopumiliotoxin 223G,Org. Lett. 2006, 8 (7), 1435-1438.
184. Bang-Guo Wei, Jie Chen, Pei-Qiang Huang,* A New Approach for the Asymmetric Syntheses of 2-epi-Deoxoprosopinine and Azasugar Derivatives, Tetrahedron 2006, 62, 190-198.
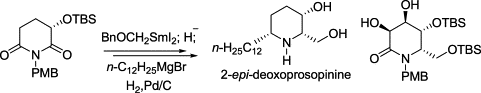
185. Xiao Zheng, Chen-Guo Feng, P.-Q. Huang,* Samarium Diiodide Promoted Asymmetric a-Amido- hydroxyalkylation of Protected 3-Hydroxy 2-carbanion Pyrrolidine, Org. Lett. 2005, 7 (4), 553-556.
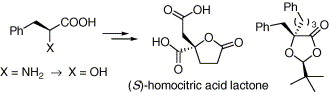
186. P.-Q. Huang,* Z.-Y. Li An Alternative Asymmetric Synthesis of (S)-Homocitric Acid Lactone Tetrahedron: Asymmetry, 2005, 16 (20), 3367-3370.
187. Yuan-Ping Ruan,* Bang-Guo Wei, Xiu-Qing Xu, Gang Liu, De-Sheng Yu, Liang-Xian Liu, Pei-Qiang Huang,* “Detailed Studies on the Enantioselective Synthesis and HPLC Enantioseparation of N-Protected 3-Hydroxyglutarimides”, Chirality 2005, 17, 595-599.
188. Tian Tang, Yuan-Ping Ruan, Jian-Liang Ye, P.-Q. Huang,* A Flexible Carbanionic Approach to Protected trans-(2R,3S)-2-Substituted 3-Aminopyrrolidines: Application to the Asymmetric Synthesis of (+)-Absouline, Synlett 2005, (2), 231-234.
189. M.-D. Chen, M.-Z. He, X. Zhou, L.-Q. Huang, Y.-P. Ruan, P.-Q. Huang,* Studies on the Diastereoselective Reductive Alkylation of (R)-Phenylglycinol Derived Phthalimide: Observation of Stereoelectronic effects, Tetrahedron, 2005, 60 (5), 1335-1344.
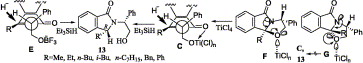
190. Zheng, J.-F.; Jin, L.-R.; Huang, P.-Q.* Enantio-divergent Synthesis of Both Enantiomers of Marine Alkaloids Haliclorensin and Isohaliclorensin, Constituent of Halitulin, Org. Lett. 2004, 6, 1139-1142;
191. Huang, P.-Q.* Lan, H.-Q.; Zheng, X.; Ruan, Y.-P. A Concise Asymmetric Synthesis of (2S,3S,7S)-3,7-Dimethylpentadecan-2-yl Acetate and Propionate, the Sex Pheromones of Pine Sawflies, J. Org. Chem. 2004, 69, 3964-3967.
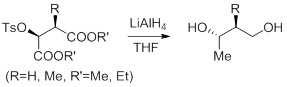
192. Liu, L.-X.; Ruan, Y.-P.; Guo, Z.-Q.; Huang, P.-Q.* A General Approach to (5S,6R)-6-Alkyl-5-benzyloxy-2-piperidinones: Application to the Asymmetric Syntheses of Neurokinin Substance P Receptor Antagonist (-)-L-733,061 and (-)-Deoxocassine, J. Org. Chem. 2004, 69, 6001– 6009;
193. P.-Q. Huang* and Jun Deng, A Flexible Approach For The Asymmetric Synthesis of N-Protected (R)-5-Alkyl tetramates and (R)-5-Alkyl tetramic Acid Derivatives, Synlett 2004, (2), 247-250;
194. M.-D. Chen, M.-Z. He, X. Zhou, L.-Q. Huang, Y.-P. Ruan and P.-Q. Huang,* Studies on the Diastereoselective Reductive Alkylation of (R)-Phenylglycinol Derived Phthalimide: Observation of Stereoelectronic effects, Tetrahedron 2004, 60(7), 1651-1657.
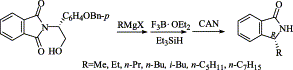
195. Jing-Xing Du, Hui-Ying Huang, P.-Q. Huang,* A New Approach to Geissman-Waiss Lactone and The Key Intermediate in the Synthesis of Pyrrolidine trans-Lactones, Tetrahedron: Asymmetry, 2004, 15 (21), 3461-3466;
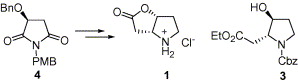
196. W.-H. Meng, T. -J. Wu, H.-K. Zhang, P.-Q. Huang,* Asymmetric Syntheses of (2S,3S,4S)-3- Hydroxy-4-methylproline And 4’-t-Butoxyamido-2’-deoxythymidine, Tetrahedron: Asymmetry, 2004, 15(24), 3899-3910.
197. P.-Q. Huang,* W.-H. Meng, A New Approach to (2S,3S,4S)-3-Hydroxy-4-methylproline, A Subunit in Echinocandin B And Related Oligopeptide Antibiotics, Lett. Org. Chem. 2004, 1(1), 99-101.
198. Tian Tang, Chen Zhu, and P.-Q. Huang,* A Flexible Approach to (S)-3-Amino-2-pyrrolidinone Derivatives, Heterocycles, 2004, 64, 121-128.
199. Y. P. Ruan, Ming-De Chen, Ming-Zhu He, Xiang Zhou, P.-Q. Huang,* A Practical Two-step Synthesis of 3-Alkyl-2,3-dihydro-1H-isoindolin-1-ones, Synth. Commun. 2004, 34(5), 853-861.
200. P.-Q. Huang,* H.-Y. Huang, An Improved Asymmetric Synthesis of Unusual Amino Acid (2S, 3S)-3-Hydroxyproline, Synth. Commun. 2004, 34(8), 1377-1382.
201. C.-G. Feng, H. Fang and P.-Q. Huang, (2TST,3TST)-3-Hydroxy-1-(4-methoxybenzyl) piperidine-2-carboxamide, Acta Cryst. 2004. E60, o1075-o1077;
202. Jian-Liang Ye, Xu Tang and P.-Q. Huang,* Further studies on the reductive–alkylation of chiral endo-himimide derived from (R)-phenylglycinol, Arkivoc 2004, ix, 34-43, at Hwww.arkat-usa.orgH;
203. Huang, P.-Q.*; Yu, X.-Y.; Liu X.-P. Asymmetric synthesis of b,g-disubstituted g-lactones, Acta Chim. Sinica 2004, 62 (18), 1794-1800 (in Chinese);
204 Ruan, Y.-P.; Xu, X.-Q.; He, M.-Z.; Zhou, X.; Huang, P.-Q* Chem. J. Chinese Universities 2004, 25 (6), 1031-1033 (in Chinese);
205. Ruan, Y.-P.; Ao, X.-P.; Zhang, X.-M.; Huang, P.-Q.* Liquid chromatographic resolution of enantiomeric 1,1 '-bi-2-naphthol and phenylsuccinic acid on a coated reversed-phase column with 2,6-O-butyled beta-cyclodextrins, Chinese J. Anal. Chem. 2004, 32 (7), 949-952 (in Chinese);
206. P.-Q. Huang,* Tian-Jun Wu and Yuan-Ping Ruan, A Flexible Approach to (S)-5-Alkyl Tetramic Acid Derivatives: Application to the Asymmetric Synthesis of (+)-Preussin and Protected (3S,4S)-AHPPA, Org. Lett. 2003, 5 (23), 4341-4344;
207. P.-Q. Huang,* L. X. Liu, B. -G. Wei, Y.-P. Ruan, Asymmetric Synthesis of (+)-L-733, 060 and (+)-CP-99, 994 Based on A New Chiral 3-Piperidinol Synthon, Org. Lett. 2003, 5 (11), 1927-1929;
208. Bi-Yan He, Tian -Jun Wu, Xian -Yong Yu, P.-Q. Huang,* A Flexible Non-amino acid-Based Formal Asymmetric Synthesis of Naturally Occurring (2R, 3S)-Aminotetradeca-5,7-dien-3-ol: Observation of a Remarkable Protecting Group Effect. Tetrahedron: Asymmetry, 2003, 14, 2101-2108.
209. P.-Q. Huang,* Bang-Guo Wei, Yuan-Ping Ruan, Asymmetric Synthesis of Antimalarial Alkaloids (+)-Febrifugine and (+)-Isofebrifugine, Synlett 2003, (11), 1663-1667;
210. P.-Q. Huang,* X. Zheng, H. Wei, Synthesis of (S)-Vasicol and (S)-3-Hydroxy-2-pyrrolidinone, Heterocycles, 2003, 60 (8), 1833-1842;
211. Huang, P.-Q.*; Wu, Tian-Jun, Ye, Jian-Liang, A Flexible Approach to the g-Amino-b-Hydroxy Acid Moiety of Hapalosin, Chin. J. Chem, 2003, 21 (7): 723-726;
212. P.-Q. Huang,* X. Zheng,, An Improved Formal Total Synthesis of (-)-Anisomycin, Arkivoc, 2003, (II), 7-14, at www.arkat-usa.org;
213 Ye J.-L., Wei Z.-B., Chen Z, Huang P.-Q.* Synthesis and crystal structure of (1R, 2S, 5R, 8S, 9R, 10S)-8-methyl-4-aza-5-phenyl-7-oxatetracyclo[8.2.1.0 (2,9).0 (4,8)]-11-en-3-one. Chin. J. Struct. Chem. 2003, 22 (2): 228-232;
214. Yu X.-Y., Cai S.-H., Chen Z.,* Huang P.-Q. NMR and ESI-MS studies on the interactions between oxodiperoxooxalatovanadate and organic ligands, Acta Chim. Sinica, 2003, 61 (7): 994-999;
215. Jin L.-R.,* Zheng J.-F., Huang S.-J., Huang P.-Q. Preparation and characterization of chiral bis (oxazolinylpyridine) nickel (II) and iron (II) complexes, Chinese J. Inorg. Chem. 2003, (11), 1207-1211.
216. X. Zheng, P-Q Huang,* Y.-P. Ruan, A. W. M. Lee and W. H. Chan, A New Approach For Asymmetric Synthesis of (R)-3-Methylpyrrolidine Alkaloids from (S)-Malic Acid,Nat. Prod. Lett. 2002, 16 (1), 53-56;
217. M.–D Chen, M.-Z. He, L.-Q. Huang, Y.-P. Ruan, P.-Q. Huang,* A Versatile Approach for the Asymmetric Synthesis of 3-Alkyl-isoindolin-1-ones, Chin. J. Chem. 2002, 20 (11): 1149-1153;
218. Zhou XW, Ye JL, Chen Z,* Chen ZW, Huang P.-Q. Studies on the interactions between bioactive peroxovanadium complexes bearing organic ligands and histidine, Acta Chim. Sinica, 2002, 60 (5): 835-840;
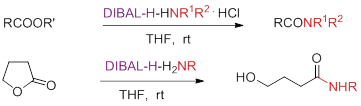
219. P.-Q. Huang,* Xiao Zheng and Xian-Ming Deng, DIBAL-H-(H2NR) and DIBAL-H-HNR1R2-HCI complexes for efficient conversion of lactones and esters to amides, Tetrahedron Lett. 2001, 42 (51), 9039-9041;
220. Huang, P.-Q.* Progress on the asymmetric synthetic methodology based on malimide, Chin. J. Org. Chem. 2001, 21(11), 1065-1073 (in Chinese);
221. Xiao Zheng, Pei Qiang Huang,* Yuan Ping Ruan, Albert W. M. Lee, and Wing Hong Chan A New Asymmetric Synthesis of (R)-3-Methylpyrrolidine Alkaloids Starting From (S)-Malic Acid, Heterocycles 2001, 55, 1505-1518.
222. Ruan Y.-P.,* Ao X.-P., Chen A.-Q., Huang P.-Q., Separation of 1,1’-binaphthalene-2,2'-diol optical isomers by derivatization high performance liquid chromatography, Chin. J Anal. Chem. 2001, 29 (12), 1398-1401;
223. J. Lin, W. H. Chan,* A. W. M. Lee,* W. Y. Wong, P. Q. Huang, Asymmetric synthesis of 1,3- and 1,3,4-substituted pyrrolidines, Tetrahedron Lett. 2000, 41 (16), 2949-2951;
224. P.-Q. Huang,* Xu Tang, An Qi Chen, An Alternative Stereoselective Synthesis of Protected Trans-5-Alkyl-4-Hydroxy-2-Pyrrolidinones, Synth. Commun. 2000, 30(13), 2259-2268;
225. Hong Kui Zhang, Quan Feng Chen, P.-Q. Huang,* A versatile Approach to protected (S)-aspartimide, (4S)-amino-2-pyrrolidinone and (3S)-aminopyrrolidine from (S)-aspartic acid, Synth. Commun. 2000, 30(13), 2431-2444;
226. Huang, P.-Q.*; Lan, H.Q. Chen, M.D.; Zhang; H.K. Some observations on the asymmetric reductive alkylation of cyclic imides, Chin. J. Org. Chem. 2000, 20(5), 790-794 (in Chinese);
227. P.-Q. Huang,* X Zheng, S L Wang, J L Ye, LR Jin and Z. Chen, A New Approach to (S)-4-Hydroxy-2-pyrrolidinone And Its 3-Substituted Analogues, Tetrahedron: Asymmetry, 1999, 10 (17), 3309-3317;
228. P.-Q. Huang,* Quan Feng Chen, Chang Lin Chen and Hong Kui Zhang, Asymmetric Synthesis of (-)-(R)-Pyrrolam A Starting From (S)-Malic Acid, Tetrahedron: Asymmetry, 1999, 10 (19), 3827-3832; correction: Tetrahedron: Asymmetry, 2000, 11, 1843
229. L.-R. Jin,* H. Wu, H.-L. Wu, P.-Q. Huang, K. Jung, H. Lim, Enantioselective Synthesis of Pyrrolydinonyl Thymine Nucleoside Analogues, Chem. Lett. 1999, 687~688;
230. Liren Jin,* Jianliang Ye, Yong Xie, Jianghong Shi, P.-Q. Huang, Kyeongeun Jung, and Hong Lim, Asymmetric Synthesis of Polyhydroxy Nucleoside Analogues from Tartaric acid, Chinese Chem. Lett. 1999, 10 (7), 543~546;
231. P.-Q. Huang,* X. Zheng, S.-L. Wang, J.- L. YE, L.-R. JIN, A New Chiral Synthesis of Naturally Occurring (-)-(S)-4-Hydroxy-2-Pyrrolidinone, Chinese Chem. Lett. 1999, 10 (9), 735-736;
232. Huang, P.-Q.* D-Quinic acid, a versatile chiron in organic synthesis, Chinese J. Org. Chem. (in Chinese) 1999, 19(4), 364-373;
233. P.-Q. Huang,* S-L Wang, J-L Ye, Y-P Ruan, Y-Q Huang, H Zheng, J-X Gao, An Easy Access to Protected (4S, 5R)-5-Alkyl-4-hydroxy-2-pyrrolidinones and their Use as Versatile Synthetic Intermediates, Tetrahedron 1998, 54(41), 12547-12560; Correction: Tetrahedron, 2000, 56, 10099
234. P.-Q. Huang,* S.-L. Wang, Y.-P Ruan, J.-X. Gao, A New Approach to (-)-Anisomycin, Nat. Prod. Lett. 1998, 11, 101-106;
235. P.-Q. Huang,* J.-L. Ye, Z. Chen, Y.-P. Ruan, J.-X. Gao, A Versatile Approach to the Activated Form of (3S, 4R)-Statine and Its Analogues, Synth. Commun. 1998, 28 (3), 417-426;
236 P.-Q. Huang,* Jing-Xing Gao, Green Chemistry: An Emerging Frontier, Progress in Chemistry, 1998, 10 (3), 265-272 (in Chinese);
237. P.-Q. Huang,* Shi-Li Wang, Hong Zheng, Xiang-Su Fei, First Asymmetric Synthesis of (2R,3R)-3-Amino-1-benzyl-2-methylpyrrolidine via A Highly Diastereroselective Reductive Alkylation, Tetrahedron Lett. 1997, 38(2), 271~272; correction: Tetrahedron Letters, 2001, 42, 363
238. P.-Q. Huang,* Xiang Su Fei; H. Zheng, a-Amidoalkylation Via Sulfones: Towards The Synthesis of Ant Venom Alkaloids, Chinese Chem. Lett. 1995, 6 (9), 739~742;
239. P.-Q. Huang,* Yuan-Ping Ruan, Synthesis of a chiral precursor for Preussin and AHPPA, Chem. J. Chinese Universities 1995, 16(12), 1914~1916 (in Chinese);
240. P.-Q. Huang; W. -S. Zhou,* Efficient Syntheses of A New Chiral Diene and A New Bridgehead Enone for A Diels-Alder Approach To Kaura-9(11),16-Dien-19-Oic Acid, Tetrahedron: Asymmetry, 1991, 2(9), 875~878;
241. P.-Q. Huang; W.-S. Zhou,* An Improved Method For The Syntheses Of Β-Substitued Cyclohexenones, Synth. Commun. 1991, 21(22), 2369~2376.
242. P.-Q. Huang; W. S. Zhou,* Synthesis of A Potential Antifeedant Sesquiterpene Intermediate From A New Terpenoid A, B-Ring Synthon By Tandem Michael Reaction, Chinese Chem. Lett. 1992, 3(10), 767~770.
243. P.-Q. Huang, K. Sabbe, M. Pottie, M. Vandewalle,* A Novel Synthesis of 19-Nor 1Α,25-Dihydroxyvitamin DB3B And Related Analogues, Tetrahedron Lett. 1995, 36 (45), 8299~8302.
244. A. Diez, P.-Q. Huang, D.-S. Grierson, H.-P. Husson,* M. Rubiralta, Preparation of A New Chiral 5,6-Dihydropyridinium Synthon, Heterocycles, 1990, 31(3), 485~492.
245. S.Arseniyadis, P.-Q. Huang, N. Morellet, J. C .Beloeil, H. -P. Husson,* On The 2, 4-Relative Stereochemistry of N-Substituted Oxazolidines Derived From Phenylglycinol, Heterocycles, 1990, 31(10), 1789-1799.
246. S. Arseniyadis, P. -Q. Huang, H. -P. Husson,* Synthesis of 2-Alkyl-Pyrrolidines: Towards Azabicyclic System, Tetrahedron Lett. 1988, 29(6), 631~634.
247. S. Arseniyadis, P. -Q. Huang And H. -P. Husson,* Short And Efficient Synthesis Of 3,5-Disubstituted Pyrrolizidine Alkaloids Via The CN(R, S) Method, Tetrahedron Lett. 1988, 29(12), 1391-1394.
248. S. Arseniyadis, P.-Q. Huang, D. Piveteau, H.-P. Husson,* Stereocontrolled Electrophilic-Nucleophilic Α,Α'-Substitution of The Pyrrolidine Ring, Tetrahedron 1988, 44(9), 2457-2470.
249. P. -Q. Huang, S. Arseniyadis, H.-P. Husson,* Chiral Pyrrolidine Synthon for A New Approach To The Synthesis of Alkaloids, Tetrahedron Lett. 1987, 28(5), 547~550.